
Head: Maria Venihaki, Assistant Professor
Background
The communication between the nervous, endocrine, and immune systems is required for the control of the immune/inflammatory response and is achieved via their common ligands, including neuropeptides and cytokines, and their receptors. The HPA axis is a major endocrine axis mediating the stress response, including the immune-inflammatory process. Stimulation of hypothalamic corticotropin-releasing-hormone (CRH) by stressful stimuli induces the expression of the proopiomelanocortin (POMC) gene in anterior pituitary corticotrophs, thus leading to increased production of corticotropin (ACTH) which in turn stimulates the synthesis and secretion of adrenal glucocorticoid, a potent antiinflammatory agent. Glucocorticoid downregulates a variety of proinflammatory factors, such as nuclear factor kappa B (NF-kB), cytokines, and adhesion molecules, whereas proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-a), interleukin-1 (IL-1) and IL-6 have been shown to directly activate the HPA axis, and thus cause increased secretion of CRH, ACTH and glucocorticoid.
CRH mRNA, peptide, and its receptor have been identified in human and rodent immune tissues such as peripheral blood cells, thymus, and spleen, as well as in inflammatory sites where it has proposed that exerts a direct proinflammatory role. In addition to the pituitary, POMC mRNA and peptide and the POMC-derived ACTH and b-endorphin have been also detected in periphery, including the immune cells, such as leucocytes.
CRH, and its receptors and POMC and POMC derived peptides such as ACTH, MSH, and b-endorphin are also expressed in mouse and human skin. CRH has been shown to induce vasodilation in skin vessels in rats an effect suggested to be mediated by mast cell degranulation. Despite the number of studies showing 1) the expression of CRH, CRH receptors, POMC and POMC-derived peptides, in human and murine skin and 2) the well established role of CRH in inflamed tissues, other than skin, their expression in wounded areas during healing and their participation in this inflammatory process have not been studied yet.
Wound healing is a highly coordinated, dynamic, and interactive process that results in the restoration of the functional integrity of the wounded tissue. Upon injury, a complex set of sequential events is set into motion, which leads to repair of the wound. Normally, healing process follows three general phases: (a) an inflammatory stage which starts immediately after the tissue injury and which involves migration of inflammatory cells such as macrophages to the site of the wound, (b) a proliferative stage which involves migration and proliferation of keratinocytes, fibroblasts, and endothelial cells and leads to re-epithelialization and formation of granulation tissue and angiogenesis, and (c) a long term tissue remodeling stage. The latter stages are highly dependent upon the earlier events of the wound healing process.
Despite the well-known beneficial effects of glucocorticoid during inflammation, several in vivo and in vitro studies have suggested that the latter, when present in pharmacological levels, inhibits wound healing in humans and animal models and increases complications in tissue repair. These effects are thought to be due to decreased cell proliferation, inhibition of collagen synthesis within fibroblasts, and failure of tissue macrophages to migrate properly in the wound.
Research
The goal of our research is to investigate the role of hormones such as CRH, POMC and glucocorticoid in wound healing process focusing also on the signaling pathways.
- Expression of CRH and its receptors and POMC and POMC-derived peptides in the skin before and during wound healing and the effect of CRH and glucocorticoid on the process of wound repair in vivo (Maria Venihaki, Olga Rassouli).
We have previously shown that peripherally expressed CRH acts as a proinflammatory factor itself and stimulates the secretion of cytokines from cells of the immune system. Proinflammatory cytokines such as IL-1a and IL-1b, and TNFa are important mediators of the process of wound healing by exerting a series of direct and indirect biological activities such as stimulation of the expression of several growth factors in the site of the wound. Recently, CRH mRNA, its receptors, and POMC mRNA and ACTH have been found in normal human and murine skin. Studies from our laboratory have also shown that CRH can act as an angiogenic factor in vivo and chemoattractant in vitro.
A series of experiments and clinical studies suggest that prolonged administration of antiinflammatory steroids leads to delayed wound healing and increased complications in tissue repair. Recent studies have demonstrated that glucocorticoid regulates the expression of various genes that encode key molecules in the wound repair process. Thus, expression of proinflammatory cytokines is suppressed in glucocorticoid-treated mice, as well as the expression of TGF-b1, 2, and 3 and their receptors, KGF, PDGF and PDGF receptors during wound healing.� These data suggest that the inhibitory effect of glucocorticoid in wound repair might be mediated, at least in part, by down-regulating the expression of the above factors.
- Effect of CRH on the most important mediators of wound healing and tissue repair process (Olga Rassouli).
CRH mRNA and peptide and CRH receptors have been identified in human fibroblasts and keratinocytes, and recently in human umbilical-vein endothelial cells. As we have previously shown CRH is a potent activator of endothelial cell migration in vitro, although the mechanisms mediating this effect of CRH are still unclear. We will evaluate the effect of CRH in the regulation of factors that play a key role in wound healing and tissue repair such as PDGF, TGF-b1, and VEGF, and adhesion molecules following skin biopsy in vivo. If a difference is found in the expression of the above factors, we will proceed with in vitro studies designed to identify the signaling pathways mediating the effect of CRH.
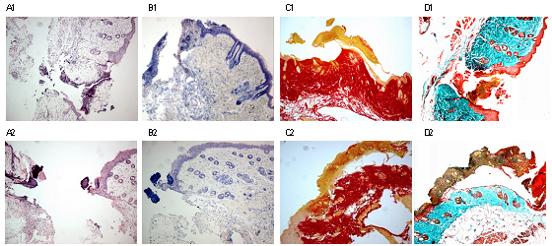
Staining of murine skin wounds
Α) H&E (leukocyte infiltration) Β) Giemsa (mast cells) C) Sirius Red (collagen) D) Masson (collagen).
1= Wild type mouse 2= Mouse with genetic deficiency of CRΗ.
Representative Publications
- Venihaki M, Gravanis A, Margioris AN. Kappa opioids exert a strong antiproliferative effect on PC12 rat pheochromocytoma cells. Peptides, 17(3): 413-419, 1996
- Venihaki M, Dikkes P, Carrigan A, and Karalis K.P. Corticotropin-releasing hormone regulates IL-6 expression during inflammation. J. Clin. Invest. 108:1159 1166, 2001
- Wlk M, Wang CC, Venihaki M, Liu J, Zhao D, Anton PM, Mykoniatis A, Pan A, Zacks J, Karalis K, Pothoulakis C. Corticotropin-releasing hormone antagonists possess anti-inflammatory effects in the mouse ileum. Gastroenterology: 123(2):505-15, 2002
- Venihaki M, Zhao J, and Karalis KP. Corticotropin-releasing hormone deficiency results in impaired splenocyte response to lipopolysaccharide. J Neuroimmunol. 141(1-2): 3-9, 2003.
- Karalis KP, Venihaki M*, Zhao J*, vanVlerken LE, Chandras C.
equally contributed authors.
NF-kappaB participates in the corticotropin-releasing, hormone-induced regulation of the pituitary proopiomelanocortin gene. J Biol Chem. 279(12):10837-40, 2004.
Funding
- Hellenic General Secretariat for Research and Technology (ΠΕΝΕΔ)
Contact
Maria Venihaki
Dept. of Clinical Chemistry-Biochemistry
School of Medicine
University of Crete
PO BOX 2208
Heraklio 71003
Crete
Greece
Tel:+30-2810-394583
Fax: +30-2810-394581
email: venihaki@med.uoc.gr