
Head: Christos Tsatsanis, Professor
Current Lab Members:
- Erini Dermitzaki, BSc, PhD (Staff Scientist)
- Eleftheria Ieronymaki, BSc, MSc (Postdoctoral Researcher)
- Maria Daskalaki, (PhD student)
- Ourania Kolliniati, (PhD student)
- Elina Paflioti, (PhD student)
- Ioanna Pantazi, (PhD student)
- Ioanna Lapi, (MSc student)
- Konstantinos Axarlis, (MSc student)
- Christina Efraimoglou, (MSc student)
- Nikol Androulaki, (BSc student)
Background
Inflammation and inflammatory diseases are complex diseases implicating various cell types including these of the innate immune system. The magnitude and type of innate immune response is regulated by factors present in the cellular microenvironment and at the intracellular level.
Insulin is a pleiotropic hormone that signals in all cells including cells of the innate immune system. Insulin, when oscillates, acts as a negative regulator of inflammation, maintaining a balanced inflammatory response. Insulin activates PI3K/Akt signaling that suppress TLR-mediated signals reducing the magnitude of the inflammatory responses. Therefore, in macrophages, the central mediator of innate immune responses, insulin acts as a negative regulator of TLR signaling.
Macrophages obtain distinct phenotypes that characterize the type and magnitude of the response to pathogenic and tissue damage signals. Such phenotypes are broadly described as being within the M1 or M2 polarization spectra, depending on whether they are hyper- or hypo-responsive to inflammatory signals. Evidence from our group has shown that Akt1 and Akt2 kinase isoforms differentially regulate macrophage responses controlling M1 and M2 polarization phenotypes (Vergadi et al, J Immunol, 2017, Vergadi et al, J. Immunol.2014, Arranz et al., PNAS, 2012 Androulidaki et al., Immunity, 2009).
Recent evidence has shown that innate immune cells, including macrophages, develop memory, also known as trained immunity. Trained immunity is shaped by infectious signals present on pathogens, such as those initiated by Pathogen Associated Molecular Patterns (PAMPs), or by non-infectious signals such as modified lipoproteins (i.e. oxidized LDL). We have recently shown that insulin signaling shapes macrophage responses and insulin resistance is a state of trained immunity (Ieronymaki et al, J. Immunol. 2019; Ieronymaki et al, Front. Immunol. 2019). Our group is currently investigating the metabolic and epigenetic mechanisms initiated by insulin signaling and insulin resistance to result in this trained immunity state.
Phagocytosis and autophagy is also altered in different states of trained immunity. Our group is currently studying the role of Akt kinases and insulin signaling in pathogen elimination through phagocytosis and autophagy. In addition, we are analyzing the differences between adult and neonatal macrophage responses in the context of phagocytosis and autophagy.
Non-coding RNAs have also been implicated in controlling innate immune responses through Akt kinases (Androulidaki at al, Immunity. 2009; Vergadi et al, J Immunol. 2014) and among those, miRNAs have been established not only as regulators of innate immune responses but also as biomarkers of inflammation. Our group, in collaboration with the Department of Translational Medicine, Lund University Medical School, is analyzing the role of miRNAs and piRNAs as biomarkers of low grade systemic inflammation in patient cohorts (Trzybulska et al, Cell Physiol Biochem. 2018; Vergadi et al, Front. Immunol. 2018; Kumar et al, Mol Cell Endocrinol. 2019).
Inflammatory responses are also regulated by metabolites originating in the gut microbiome as well as by metabolites and small molecules made available through nutrition. Our group utilizes cell culture and mouse models of inflammatory diseases such as obesity/type 2 diabetes, Inflammatory bowel disease, inflammation-induced intestinal tumorigenesis or skin inflammation, to understand the crosstalk of the gut microbiome, nutrition and metabolic products in macrophage responses and inflammation. Recent evidence from our group has shown that terpenes derived from the algae Laurencia promote M2-type of macrophage responses suppressing inflammatory bowel disease in mice (Daskalaki et al, Mar. Drugs. 2019).
Research
Metabolic and epigenetic mechanisms regulating macrophage responses; the role of insulin signaling and Akt kinases.
We have recently shown that macrophages become insulin resistance and obtain an M2-like phenotype with distinct TLR responses, describing a state of trained immunity (Ieronymaki et al, J. Immunol. 2019; Ieronymaki et al, Front. Immunol. 2019). We are currently investigating the epigenetic mechanisms involved in macrophage training in the context of either genetically-induced insulin resistance (Akt2 deficient or IGF1R deficient macrophages) or macrophages derived from diet-induced insulin resistant mice. The interplay between epigenetic alterations at the level of histone modifications and cell metabolism is being investigated. The role of autophagy and phagocytosis in the context of insulin resistance-trained macrophages is also analyzed.
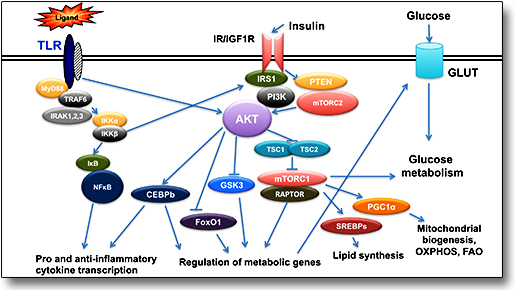
Regulation of macrophage responses in the context of critical illness and sepsis and the role of insulin signaling (in collaboration with Associate Prof. K. Vaporidi, Univ. of Crete).
Critically ill and septic patients enter a state of trained immunity, also characterized as endotoxin tolerance or immunoparalysis. We have previously shown that Akt kinases and miRNAs play a key role in regulating endotoxin tolerance (Androulidaki et al, Immunity 2009; Doxaki et al, J Immunol. 2015, Vergadi et al, Front. Immunol. 2018). Insulin is continuously infused in critically ill patients and insulin oscillation is abolished. We are currently investigating the contribution of insulin oscillatory signaling and insulin resistance in regulating macrophage responses and immunoparalysis, using mouse models and ex-vivo studies from patient samples.
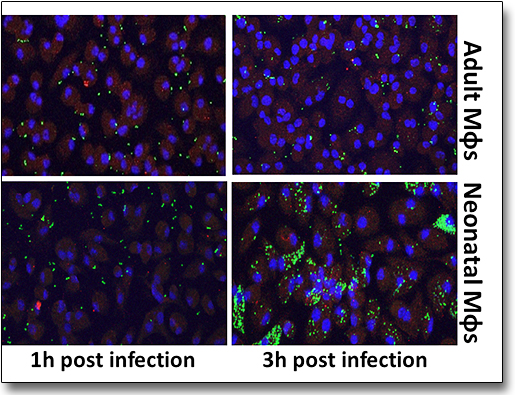
Understanding differences between adult and neonatal innate immune responses (in collaboration with Assistant Prof. E. Vergadi, Dept. of Pediatrics Univ. of Crete, Prof. T. Chavakis, TU Dresden, Prof. G. Hajishengallis, Univ. of Pennsylvania).
Newborns rely on innate immunity to combat pathogens prior to maturation of the adaptive immune system, yet innate immune responses are still immature and not always effective. Thus, neutrophil recruitment at the site of inflammation is not efficient and the capacity of neonatal macrophages to eliminate bacteria is not as efficient as it is in adult cells. We are currently investigating differences between adult and neonatal innate immune responses in the context of neutrophil recruitment and the role of Del-1 protein during polymicrobial sepsis. We are also studying the role of autophagy as a mechanism of pathogen clearance in neonates and the role of Akt kinases in this context.
The crosstalk of gut microbiome and innate immune responses and the role of dietary and metabolic products (in collaboration with Associate Prof. M. Venihaki).
It is widely acknowledged that the gut microbiome affects innate immune responses in the context of metabolic and inflammatory diseases. Our group analyzes the impact of insulin resistance-trained macrophages in shaping the gut microbiome using mice carrying genetically-induced insulin resistant macrophages. We are also studying the impact of nutritional products and metabolites in shaping the gut microbiome and how these can affect macrophage responses and the development of inflammatory diseases. We have recently identified algae-derived terpenes and other molecules having potent anti-inflammatory activity. We have developed a cell culture platform utilizing adipocytes and macrophages, for screening isolated compounds or extracts from marine organisms and validate those in in vivo models of inflammatory diseases (obesity/type 2 diabetes, inflammatory bowel disease, skin inflammation and other) to identify potential bioactive molecules or extracts.
Regulation of adipocyte function and their contribution in metabolic inflammation.
Metabolic inflammation is controlled by a complex interaction of adipocytes and immune cells infiltrating the adipose tissue. Central players of this crosstalk are macrophages and adipocytes, which share some biological properties. We are investigating how nutritional metabolites can affect adipocyte differentiation and function and the contribution of cytokines and Akt signaling in these processes, in the context of metabolic inflammation and obesity.
Circulating ncRNAs (miRNAs and piRNAs) as biomarkers of inflammatory diseases (in collaboration with Lund University).
Circulating miRNAs are promising biomarkers for disease diagnosis and stratification. Our group has identified miRNAs as contributors of inflammatory disease pathologies and we are currently investigating the potential value of these miRNAs as biomarkers. We have recently shown that serum miR-155 is a potential biomarker of male subfertility (Tsatsanis et al, Hum Rep, 2015; Trzybulska et al, J Assist Reprod Genet. 2017; Trzybulska et al, Cell Physiol Biochem. 2018). We are currently investigating the potential of serum ncRNAs as biomarkers of different inflammatory conditions including sepsis, acute renal injury and metabolic inflammation as well as male fertility. The crosstalk of sex hormones with metabolic inflammation and miRNA expression is also investigated.
Selected Publications
1. Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009 Aug 21;31(2):220-31 (indexed as ‘Highly cited paper’-ISI)2. Zacharioudaki V, Androulidaki A, Arranz A, Margioris AN and Tsatsanis C. Adiponectin promotes endotoxin tolerance in macrophages by inducing IRAK-M. J. Immunol. 2009 182(10):6444-51
3. Arranz A., Doxaki C., Vergadi E., Martinez Y., Vaporidi K., Vanihaki M., Lagoudaki E., Stathopoulos E., Margioris A., Tsichlis PN and Tsatsanis C. Akt1 and Akt2 differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. USA, 2012 109(24):9517-22
4. Karakasilioti I, Kamileri I, Chatzinikolaou G, Kosteas T, Vergadi E, Robinson AR, Tsamardinos I, Rozgaja TA, Siakouli S, Tsatsanis C, Niedernhofer LJ, Garinis GA. DNA damage triggers a chronic auto-inflammatory response leading to fat depletion in NER progeria. Cell Metab. 2013, 18(3):403-15
5. E. Vergadi, K Vaporidi, E Theodorakis, C Doxaki, E Lagoudaki, E Ieronymaki, V I. Alexaki, M Helms, E Kondili, B Soennichsen, E N. Stathopoulos, A N. Margioris; D Georgopoulos and C Tsatsanis Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction J. Immunol. 2014;192(1):394-406
6. Doxaki C, Kampranis SC, Eliopoulos AG, Spilianakis C and C. Tsatsanis. Coordinated Regulation of miR-155 and miR-146a Genes during Induction of Endotoxin Tolerance in Macrophages. J Immunol. 2015 Dec 15;195(12):5750-61
7. Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J Immunol. 2017 Feb 1;198(3):1006-1014. (indexed as ‘Highly cited paper’-ISI)
8. Lyroni K, Patsalos A, Daskalaki MG, Doxaki C, Soennichsen B, Helms M, Liapis I, Zacharioudaki V, Kampranis SC, Tsatsanis C. Epigenetic and Transcriptional Regulation of IRAK-M Expression in Macrophages. J Immunol. 2017 Feb 1;198(3):1297-1307.
9. Silva MC, Davoli-Ferreira M, Medina TS, Sesti-Costa R, Silva GK, Lopes CD, Cardozo LE, Gava FN, Lyroni K, Dias FC, Frade AF, Baron M, Nakaya HI, Figueiredo F, Alves-Filho JC, Cunha FQ, Tsatsanis C, Chevillard C, Cunha-Neto E, Hirsch E, Silva JS, Cunha TM. Canonical PI3Kγ signaling in myeloid cells restricts Trypanosoma cruzi infection and dampens chagasic myocarditis. Nat Commun. 2018 Apr 17;9(1):1513.
10. Alexaki VI, Fodelianaki G, Neuwirth A, Mund C, Kourgiantaki A, Ieronimaki E, Lyroni K, Troullinaki M, Fujii C, Kanczkowski W, Ziogas A, Peitzsch M, Grossklaus S, Sönnichsen B, Gravanis A, Bornstein SR, Charalampopoulos I, Tsatsanis C, Chavakis T. DHEA inhibits acute microglia-mediated inflammation through activation of the TrkA-Akt1/2-CREB-Jmjd3 pathway. Mol Psychiatry. 2018 Jun;23(6):1410-1420
11. Vergadi E, Vaporidi K, Tsatsanis C. Regulation of Endotoxin Tolerance and Compensatory Anti-inflammatory Response Syndrome by Non-coding RNAs. Front Immunol. 2018 Nov 20;9:2705.
12. Daskalaki MG, Vyrla D, Harizani M, Doxaki C, Eliopoulos AG, Roussis V, Ioannou E, Tsatsanis C, Kampranis SC. Neorogioltriol and Related Diterpenes from the Red Alga Laurencia Inhibit Inflammatory Bowel Disease in Mice by Suppressing M1 and Promoting M2-Like Macrophage Responses. Mar Drugs. 2019 Feb 2;17(2). pii: E97
13. Ieronymaki E, Theodorakis EM, Lyroni K, Vergadi E, Lagoudaki E, Al-Qahtani A, Aznaourova M, Neofotistou-Themeli E, Eliopoulos AG, Vaporidi K, Tsatsanis C. Insulin Resistance in Macrophages Alters Their Metabolism and Promotes an M2-Like Phenotype. J Immunol. 2019 Mar 15; 202(6):1786-1797.
14. Ieronymaki E, Daskalaki MG, Lyroni K, Tsatsanis C. Insulin Signaling and Insulin Resistance Facilitate Trained Immunity in Macrophages Through Metabolic and Epigenetic Changes. Front Immunol. 2019 Jun 12;10:1330.
Currently active grants
1. Hellenic Ministry of Education Grant EΔBM34-Research Teams (2017-2019) IMMUNAKT
2. Hellenic Secreteriat for Research Grant on Infrastructures (2017-2020) CMBR
3. Hellenic Secreteriat for Research Grant Ερευνώ-Καινοτομώ (2018-2021) MedSUSHI
4. Hellenic Secreteriat for Research Grant Ερευνώ-Καινοτομώ (2018-2021) iFUNFoods
5. EU H2020 BBI program (2018-2022) AQUABIOPROFIT
6. Hellenic Foundation for Research Innovation (ΕΛΙΔΕΚ) (2018-2021) (In collaboration with Dr. Eleni Vergadi, Asistant Professor of Pediatrics) NeoPhagES
Contact
Christos TSATSANIS(Curriculum Vitae)
Dept. of Clinical Chemistry-Biochemistry
School of Medicine
University of Crete
PO BOX 2208
Heraklio 71003
Crete
Greece
Tel:+30-2810-394833
Fax: +30-2810-394581
email: tsatsani@med.uoc.gr